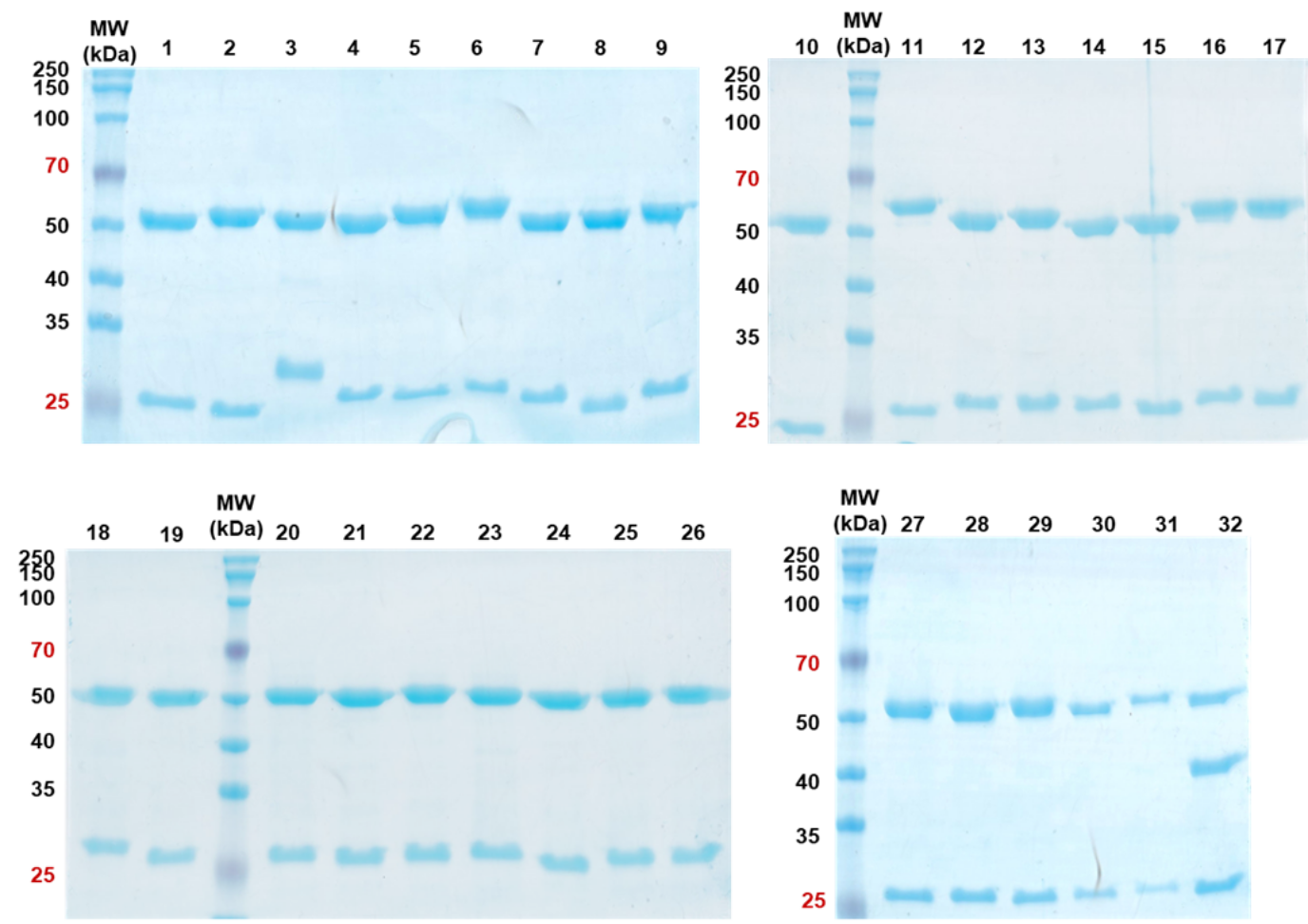
Sds Page Reducing Conditions
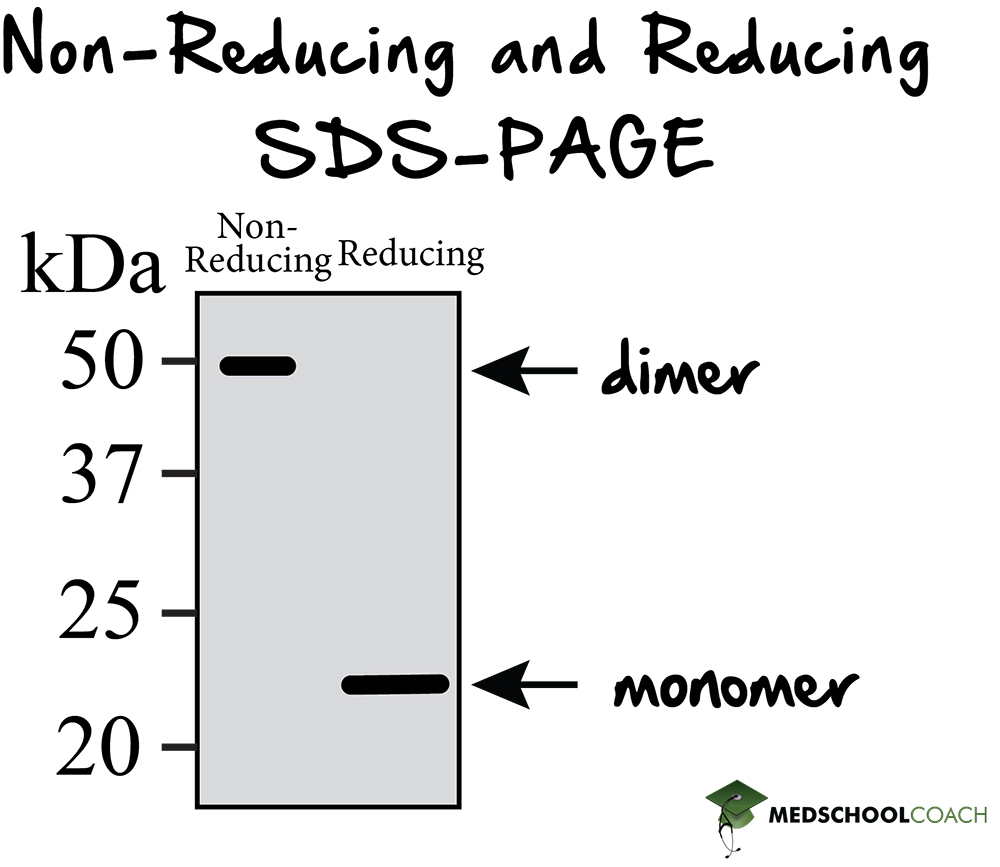
Sds Page Reducing Conditions - A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. By heating the sample under denaturing and reducing conditions, proteins become unfolded and. If we had a heterotrimer, we would only see one band.
If we had a heterotrimer, we would only see one band. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. By heating the sample under denaturing and reducing conditions, proteins become unfolded and.
By heating the sample under denaturing and reducing conditions, proteins become unfolded and. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. If we had a heterotrimer, we would only see one band.
Reducing Sds Page And Non Reducing Sds Page Analysis Of Purified Hprl
By heating the sample under denaturing and reducing conditions, proteins become unfolded and. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. If we had a heterotrimer, we would only see one band.
High Throughput Antibody Production ProteoGenix
A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. If we had a heterotrimer, we would only see one band. By heating the sample under denaturing and reducing conditions, proteins become unfolded and.
Reducing SDSPAGE and nonreducing SDSPAGE analysis of purified hPRL
A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. By heating the sample under denaturing and reducing conditions, proteins become unfolded and. If we had a heterotrimer, we would only see one band.
Gel Electrophoresis, PAGE, SDS PAGE MCAT Biochemistry MedSchoolCoach
By heating the sample under denaturing and reducing conditions, proteins become unfolded and. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. If we had a heterotrimer, we would only see one band.
SDSPAGE of IgG and F(ab’) 2 preparations. Protein samples in SDS
If we had a heterotrimer, we would only see one band. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. By heating the sample under denaturing and reducing conditions, proteins become unfolded and.
SDS PAGE tutorial Biochemistry Pinterest Chemistry
If we had a heterotrimer, we would only see one band. By heating the sample under denaturing and reducing conditions, proteins become unfolded and. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not.
SDS (20)PAGE analysis under reducing conditions of monoclonal IgG
If we had a heterotrimer, we would only see one band. By heating the sample under denaturing and reducing conditions, proteins become unfolded and. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not.
SDSPAGE under reducing (A) and nonreducing conditions (B) of
By heating the sample under denaturing and reducing conditions, proteins become unfolded and. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. If we had a heterotrimer, we would only see one band.
SDSPAGE showing protein release (reducing conditions), cellular uptake
If we had a heterotrimer, we would only see one band. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. By heating the sample under denaturing and reducing conditions, proteins become unfolded and.
SDSPAGE and western blot analysis of VHHFc seed extracts. About 32 µg
By heating the sample under denaturing and reducing conditions, proteins become unfolded and. If we had a heterotrimer, we would only see one band. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not.
By Heating The Sample Under Denaturing And Reducing Conditions, Proteins Become Unfolded And.
If we had a heterotrimer, we would only see one band. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not.