Vsepr Cheat Sheet
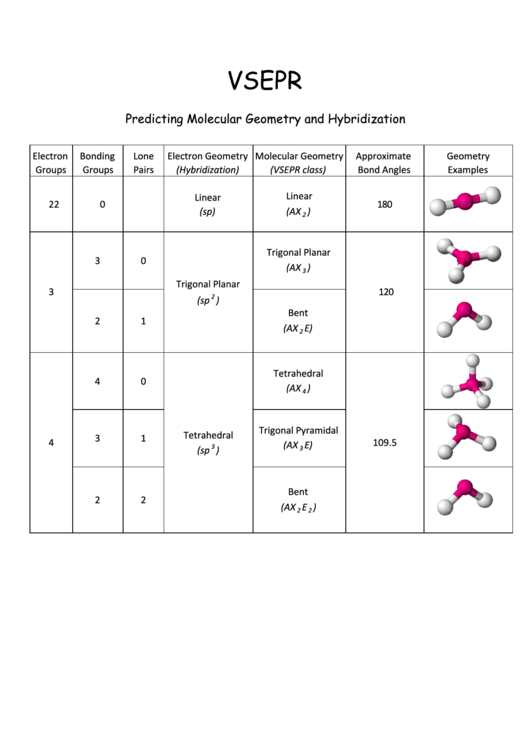
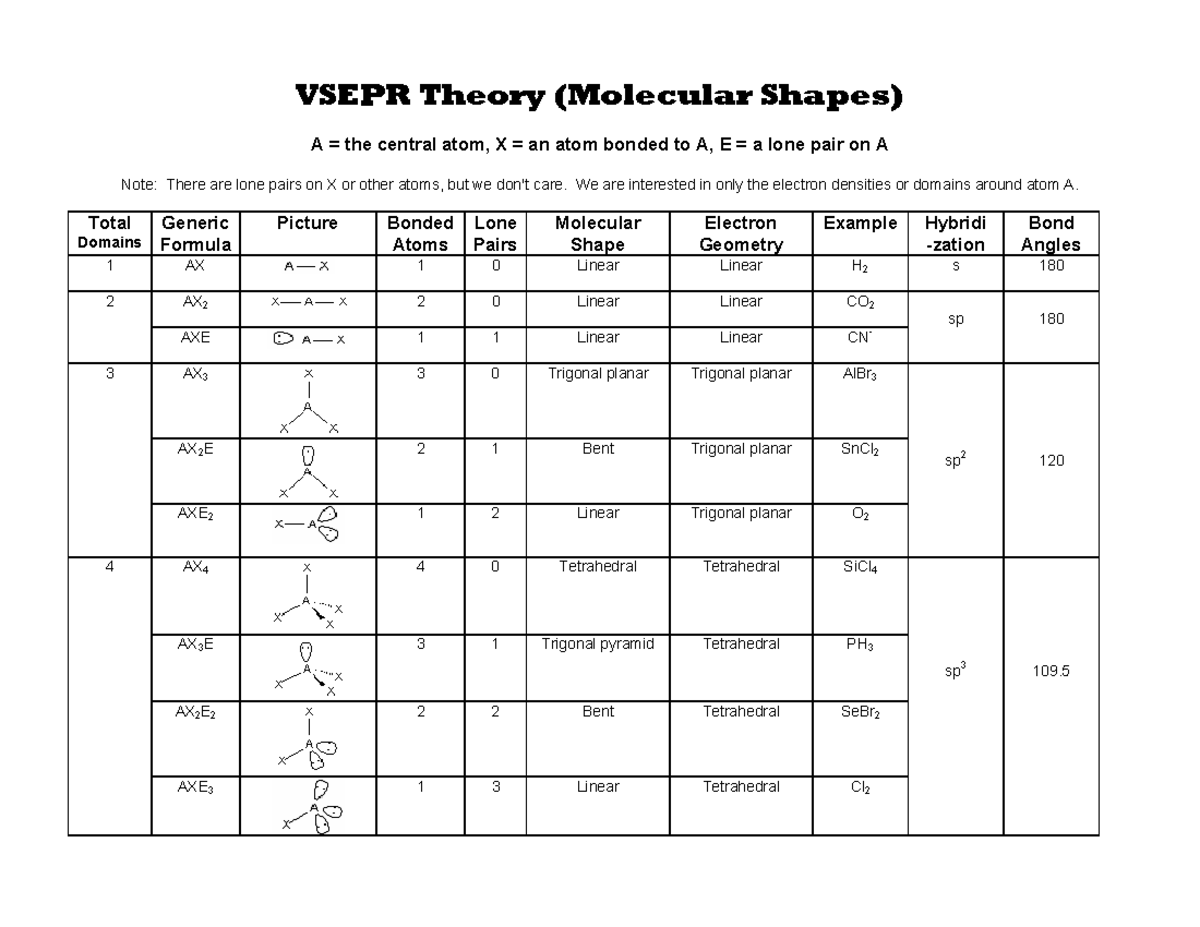
Vsepr Cheat Sheet - We are interested in only the electron densities or domains around atom a. Web using vsepr to predict the shapes of molecules “electron groups” include bonds, lone pairs, and odd (unpaired) electrons. There are lone pairs on x or other atoms, but we don't care. It is based on the assumption that pairs of electrons occupy space, and. A multiple bond (double bond or triple bond) counts as one electron group. Web a = the central atom, x = an atom bonded to a, e = a lone pair on a note:
There are lone pairs on x or other atoms, but we don't care. Web using vsepr to predict the shapes of molecules “electron groups” include bonds, lone pairs, and odd (unpaired) electrons. We are interested in only the electron densities or domains around atom a. A multiple bond (double bond or triple bond) counts as one electron group. Web a = the central atom, x = an atom bonded to a, e = a lone pair on a note: It is based on the assumption that pairs of electrons occupy space, and.
There are lone pairs on x or other atoms, but we don't care. Web using vsepr to predict the shapes of molecules “electron groups” include bonds, lone pairs, and odd (unpaired) electrons. A multiple bond (double bond or triple bond) counts as one electron group. We are interested in only the electron densities or domains around atom a. It is based on the assumption that pairs of electrons occupy space, and. Web a = the central atom, x = an atom bonded to a, e = a lone pair on a note:
Pin by Siphora Ketchakeu on organic chemistry 1 Teaching chemistry
A multiple bond (double bond or triple bond) counts as one electron group. Web a = the central atom, x = an atom bonded to a, e = a lone pair on a note: We are interested in only the electron densities or domains around atom a. There are lone pairs on x or other atoms, but we don't care..
Lewis structuresvsepr theory cheat sheet Vsepr Theory, Polyatomic Ion
There are lone pairs on x or other atoms, but we don't care. It is based on the assumption that pairs of electrons occupy space, and. Web using vsepr to predict the shapes of molecules “electron groups” include bonds, lone pairs, and odd (unpaired) electrons. We are interested in only the electron densities or domains around atom a. A multiple.
Vsepr Theory Summary Chart Download Printable Pdf Templateroller Images
There are lone pairs on x or other atoms, but we don't care. It is based on the assumption that pairs of electrons occupy space, and. Web using vsepr to predict the shapes of molecules “electron groups” include bonds, lone pairs, and odd (unpaired) electrons. We are interested in only the electron densities or domains around atom a. Web a.
Química 12º 2.0 Tema 3 Enlace químico
There are lone pairs on x or other atoms, but we don't care. We are interested in only the electron densities or domains around atom a. It is based on the assumption that pairs of electrons occupy space, and. Web a = the central atom, x = an atom bonded to a, e = a lone pair on a note:.
Geometry Math geometry, Math cheat sheet, Basic math
Web a = the central atom, x = an atom bonded to a, e = a lone pair on a note: There are lone pairs on x or other atoms, but we don't care. We are interested in only the electron densities or domains around atom a. Web using vsepr to predict the shapes of molecules “electron groups” include bonds,.
PPT Covalent Bonding PowerPoint Presentation, free download ID590204
Web a = the central atom, x = an atom bonded to a, e = a lone pair on a note: We are interested in only the electron densities or domains around atom a. Web using vsepr to predict the shapes of molecules “electron groups” include bonds, lone pairs, and odd (unpaired) electrons. A multiple bond (double bond or triple.
XeO2F2 Lewis Structure, Geometry, Hybridization, and Polarity
Web using vsepr to predict the shapes of molecules “electron groups” include bonds, lone pairs, and odd (unpaired) electrons. There are lone pairs on x or other atoms, but we don't care. Web a = the central atom, x = an atom bonded to a, e = a lone pair on a note: It is based on the assumption that.
Molecular geometry cheat sheet
There are lone pairs on x or other atoms, but we don't care. Web using vsepr to predict the shapes of molecules “electron groups” include bonds, lone pairs, and odd (unpaired) electrons. It is based on the assumption that pairs of electrons occupy space, and. We are interested in only the electron densities or domains around atom a. Web a.
Vsepr handout vesper cheat sheet VSEPR Theory (Molecular Shapes) A
We are interested in only the electron densities or domains around atom a. It is based on the assumption that pairs of electrons occupy space, and. There are lone pairs on x or other atoms, but we don't care. A multiple bond (double bond or triple bond) counts as one electron group. Web using vsepr to predict the shapes of.
Vsepr Chart
We are interested in only the electron densities or domains around atom a. It is based on the assumption that pairs of electrons occupy space, and. There are lone pairs on x or other atoms, but we don't care. Web using vsepr to predict the shapes of molecules “electron groups” include bonds, lone pairs, and odd (unpaired) electrons. Web a.
It Is Based On The Assumption That Pairs Of Electrons Occupy Space, And.
There are lone pairs on x or other atoms, but we don't care. Web a = the central atom, x = an atom bonded to a, e = a lone pair on a note: We are interested in only the electron densities or domains around atom a. A multiple bond (double bond or triple bond) counts as one electron group.